No products in the cart.

MASSACHUSETTS Marijuana Packaging and Labeling State Law
Massachusetts’ cannabis labeling and packaging guidelines include the following:
(E) Packaging and Labeling
(1) Marijuana shall be packaged in plain, opaque, tamper-proof, and child-proof containers without depictions of the product, cartoons, or images other than the RMD’s logo. Edible MIPs shall not bear a reasonable resemblance to any product available for consumption as a commercially available candy.
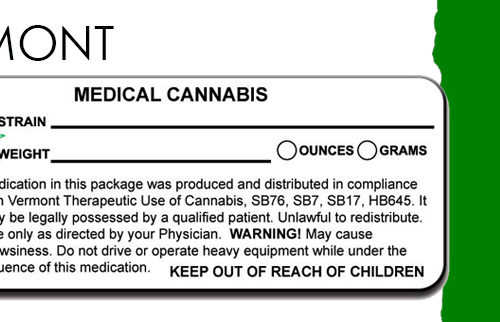
(2) Labeling of Marijuana (Excluding MIPs). The RMD shall place a legible, firmly affixed label on which the wording is no less than 1/16 inch in size on each package of marijuana that it prepares for dispensing, containing at a minimum the following information
(a) The registered qualifying patient’s name;
(b) The name and registration number of the RMD that produced the marijuana, together with the RMD’s telephone number and mailing address, and website information, if any;
(c) The quantity of usable marijuana contained within the package;
(d) The date that the RMD packaged the contents;
(e) A batch number, sequential serial number, and bar code when used, to identify the batch associated with manufacturing and processing;
(f) The cannabinoid profile of the marijuana contained within the package, including THC level;
(g) A statement that the product has been tested for contaminants, that there were no adverse findings, and the date of testing in accordance with 105 CMR 725.105(C)(2); and
(h) This statement, including capitalization: “This product has not been analyzed or approved by the FDA. There is limited information on the side effects of using this product, and there may be associated health risks. Do not drive or operate machinery when under the influence of this product. KEEP THIS PRODUCT AWAY FROM CHILDREN.”
(3) Labeling of MIPs. The RMD shall place a legible, firmly affixed label on which the wording is no less than 1/16 inch in size on each MIP that it prepares for dispensing, containing at a minimum the following information:
(a) The registered qualifying patient’s name;
(b) The name and registration number of the RMD that produced the MIP, together with the RMD’s telephone number and mailing address, and website information, if any;
(c) The name of the product;
(d) The quantity of usable marijuana contained within the product as measured in ounces;
(e) A list of ingredients, including the cannabinoid profile of the marijuana contained within the product, including the THC level;
(f) The date of product creation and the recommended “use by” or expiration date;
(g) A batch number, sequential serial number, and bar code when used, to identify the batch associated with manufacturing and processing;
(h) Directions for use of the product if relevant;
(i) A statement that the product has been tested for contaminants, that there were no adverse findings, and the date of testing in accordance with 105 CMR 725.105(C)(2);
(j) A warning if nuts or other known allergens are contained in the product; and
(k) This statement, including capitalization: “This product has not been analyzed or approved by the FDA. There is limited information on the side effects of using this product, and there may be associated health risks. Do not drive or operate machinery when under the influence of this product. KEEP THIS PRODUCT AWAY FROM CHILDREN.”
For more information, please refer to the Implementation of an Act for the Humanitarian Medical Use of Marijuana.