No products in the cart.

NEVADA Marijuana Packaging and Labeling State Law
Nevada’s cannabis labeling and packaging guidelines include the following:
NAC 453A.500 Packaging: Generally. (NRS 453A.370)
1. Any product containing marijuana must be packaged in child-resistant packaging in accordance with 16 C.F.R. § 1700 or the standards specified in subsection 2 or 3.
2. Except as otherwise provided in subsection 3, marijuana-infused products in solid or liquid form must be packaged in plastic which is 4 millimeters or more in thickness and must be heat-sealed without an easy-open tab, dimple, corner or flap so that it is difficult for a child to open and as a tamperproof measure.
3. Marijuana-infused products in liquid form may be sealed using a metal crown cork-style bottle cap.
4. Any container or packaging containing usable marijuana, edible marijuana products or marijuana-infused products must protect the contents from contamination and must not impart any toxic or deleterious substance to the usable marijuana or marijuana product.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
NAC 453A.502 Labeling: Generally. (NRS 453A.370) Each cultivation facility, facility for the production of edible marijuana products or marijuana-infused products and medical marijuana dispensary shall:
1. Use for labeling all marijuana, edible marijuana products and marijuana-infused products the standard label described in NAC 453A.506 to 453A.512, inclusive;
2. Exercise strict control over labeling materials issued for use in labeling operations for marijuana, edible marijuana products and marijuana-infused products;
3. Carefully examine labeling materials issued for a batch for identity and conformity to the labeling specified in the applicable production or control records; and
4. Have and follow written procedures describing in sufficient detail the control procedures employed for the issuance of labeling.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
NAC 453A.504 Labeling as “organic.” (NRS 453A.370) A cultivation facility or facility for the production of edible marijuana products or marijuana-infused products shall not label usable marijuana, edible marijuana products or marijuana-infused products as “organic” unless the marijuana plants used are produced, processed and certified in a manner that is consistent with the national organic standards established by the United States Department of Agriculture in accordance with the Organic Foods Production Act of 1990.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
NAC 453A.506 Maximum unit size; minimum requirements for font and size of label. (NRS 453A.370)
1. Any medical marijuana establishment that packages marijuana, edible marijuana products or marijuana-infused products must individually package, label and seal the marijuana or marijuana products in unit sizes such that no single unit contains more than a 2 1/2 ounce supply of marijuana.
2. For marijuana, edible marijuana products or marijuana-infused products that are intended to be dispensed or sold to a holder of a valid registry identification card or his or her designated primary caregiver:
(a) The text used on all labeling must be printed in at least 10-point font and may not be in italics; and
(b) Each label must be at least 2 3/4 inches high by 4 inches wide.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
NAC 453A.508 Labeling requirements for marijuana and related products for sale to medical marijuana dispensary. (NRS 453A.370)
1. A cultivation facility or facility for the production of edible marijuana products or marijuana-infused products shall label all marijuana, edible marijuana products and marijuana-infused products before it sells the marijuana or marijuana products to a medical marijuana dispensary and shall securely affix to the package a label that includes, without limitation, in legible English:
(a) The name of the medical marijuana establishment and its medical marijuana establishment registration certificate number;
(b) The lot number;
(c) The date of harvest;
(d) The date of final testing;
(e) The date on which the product was packaged;
(f) The cannabinoid profile and potency levels and terpinoid profile as determined by the independent testing laboratory;
(g) If the product is perishable, the expiration date; and
(h) The quantity of marijuana being sold.
2. The label required by subsection 1 for a container or package containing usable marijuana, edible marijuana products or marijuana-infused products sold by a cultivation facility or facility for the production of edible marijuana products or marijuana-infused products must be in substantially the following form:
| JT’S NURSERY
Certificate Number: 123 456 789 001 0001 Lot Number: 1234 Harvested on: 01/01/2013 Final Testing Date: 01/15/2013 Packaged on: 01/17/2013 Best if used by: March 17, 2013 16.7% THC 1.5% 0.3% CBN Myrcene 5.6 mg/g Limonene 5.1 mg/g Valencene 3.5 mg/g Net Weight: 2 lbs. |
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
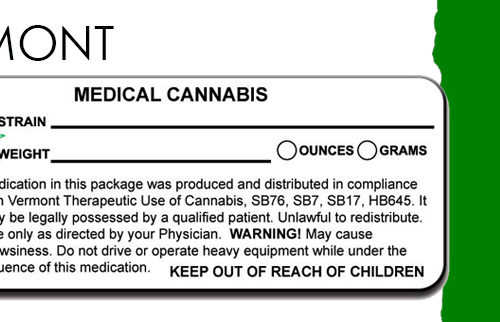
NAC 453A.510 Labeling requirements for usable marijuana sold at retail; accompanying materials. (NRS 453A.370)
- A medical marijuana dispensary must affix to each container or package containing usable marijuana sold at retail a label which must include, without limitation:
Adopted Regulation R148-15
(a) The business or trade name and the medical marijuana establishment registration certificate number of the cultivation facility that cultivated and sold the usable marijuana.
(b) The lot number.
(c) The date and quantity dispensed, including the net weight measured in ounces and grams or by volume, as appropriate.
(d) The name and registry identification card number of the patient and, if applicable, the name of his or her designated primary caregiver, or, if the patient holds a letter of approval, the name of the patient and the name and number of the registry identification card of his or her designated primary caregiver.
(e) The name and address of the medical marijuana dispensary.
(f) The cannabinoid profile and potency levels and terpenoid profile as determined by the independent testing laboratory, which may include the potential total THC but shall not include any other calculated level of THC.
(g) A warning that states: “This product may have intoxicating effects and may be habit forming.”
(h) The statement: “This product may be unlawful outside of the State of Nevada.”
(i) The date on which the marijuana was harvested.
- The label required by subsection 1 for a container or package containing usable marijuana sold at retail must be in substantially the following form:
| Joe’s Plant Emporium Cert.#: 123 456 789 001 0001
Lot#: 1234 Harvested: 01/01/2013 Dispensed to: John J. Smith #1234987 on 11/27/2013 by We Care Dispensary 123 Main Street, Carson City, NV 89701 WARNING: This product may have intoxicating effects and may be habit forming. 16.7% THC 1.5% 0.3% CBN Myrcene 5.6 mg/g Limonene 5.1 mg/g Valencene 3.5 mg/g Net Weight: .25 ounces (7 grams) This product may be unlawful outside the State of Nevada. |
3. A medical marijuana dispensary must provide with all usable marijuana sold at retail accompanying material that discloses any pesticides applied to the marijuana plants and growing medium during production and processing and contains the following warnings:
(a) “Warning: This product may have intoxicating effects and may be habit forming. Smoking is hazardous to your health.”
(b) “There may be health risks associated with consumption of this product.”
(c) “Should not be used by women who are pregnant or breast feeding.”
(d) “For use only by the person named on the label of the dispensed product. Keep out of the reach of children.”
(e) “Marijuana can impair concentration, coordination and judgment. Do not operate a vehicle or machinery under the influence of this drug.”
4. The text used on all accompanying material must be printed in at least 12-point font and may not be in italics.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
NAC 453A.512 Labeling requirements for edible marijuana products or marijuana-infused products sold at retail; accompanying materials. (NRS 453A.370)
1. A medical marijuana dispensary must affix to each container or package containing edible marijuana products or marijuana-infused products sold at retail a label which must include, without limitation:
(a) The business or trade name and the medical marijuana establishment registration certificate number of the facility for the production of edible marijuana products or marijuana-infused products that manufactured and sold the product.
(b) The lot numbers of all marijuana used to create the product.
(c) The batch number of the product.
(d) The date and quantity dispensed, including the net weight in ounces and grams or by volume, as appropriate.
(e) The name and registry identification card number of the patient and, if applicable, the name of his or her designated caregiver.
(f) The name and address of the medical marijuana dispensary.
(g) The date on which the product was manufactured.
(h) If the product is perishable, a suggested use-by date.
(i) The total milligrams of active cannabinoids and terpinoids in the product, as provided by the independent testing laboratory that tested the product.
(j) A list of all ingredients and all major food allergens as identified in 21 U.S.C. §§ 343.
(k) A warning that states: “Caution: When eaten or swallowed, the intoxicating effects of this drug may be delayed by 2 or more hours.”
(l) If a marijuana extract was added to the product, a disclosure of the type of extraction process and any solvent, gas or other chemical used in the extraction process, or any other compound added to the extract.
(m) A warning that states: “This product may have intoxicating effects and may be habit forming.”
(n) A statement that: “This product may be unlawful outside of the State of Nevada.”
2. The front and back of the label required by subsection 1 for a container or package containing edible marijuana products or marijuana-infused products sold at retail must be in substantially the following form:
| We Care Dispensary, 123 Main Street, Carson City, NV 89701
Date Dispensed: 3/27/2014 To: John J. Smith #1234987 Cookie Net Weight: 6oz (168 Grams) Serving Size: 10mg of THC Contains 10 servings and a total of 100 MG of THC Use by: 6/3/2014 Myrcene 5.6 mg/g Limonene 5.1 mg/g Valencene 3.5 mg/g CAUTION: When eaten or swallowed the intoxicating effects of this product can be delayed 2 or more hours. This product may be unlawful outside the State of Nevada. |
| Manufactured at: Joe’s Kitchen Cert.#: 321654987101 0401
123 Main Street, Las Vegas, NV on 2/1/14 Lot#: 1234 Batch #5463 INGREDIENTS: Flour, Butter, Canola Oil, Sugar, Chocolate, Marijuana, Strawberries CONTAINS ALLERGENS: Milk, Wheat Contains marijuana extract processed with butane. WARNING: This product may have intoxicating effects and may be habit forming. |
3. A medical marijuana dispensary must provide with all edible marijuana products and marijuana-infused products sold at retail accompanying material that discloses any pesticides applied to the marijuana plants and growing medium during production of the marijuana used to create the extract added to the edible marijuana products or marijuana-infused products and the type of extraction method used, including, without limitation, any solvents, gases or other chemicals or compounds used to produce or that are added to the extract, and contains the following warnings:
(a) “There may be health risks associated with consumption of this product.”
(b) “This product contains or is infused with marijuana or active compounds of marijuana.”
(c) “Should not be used by women who are pregnant or breast feeding.”
(d) “For use only by the person named on the label of the dispensed product. Keep out of the reach of children.”
(e) “Products containing marijuana can impair concentration, coordination and judgment. Do not operate a vehicle or machinery under the influence of this drug.”
(f) “Caution: When eaten or swallowed, the intoxicating effects of this drug may be delayed by 2 or more hours.”
4. The text used on all accompanying material must be printed in at least 12-point font and may not be in italics.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
NAC 453A.514 Required examinations of packaged and labeled products. (NRS 453A.370) Each cultivation facility, facility for the production of edible marijuana products or marijuana-infused products and medical marijuana dispensary shall:
1. Examine packaged and labeled products during finishing operations to provide assurance that the containers and packages have the correct labels;
2. Collect a representative sample of units at the completion of finishing operations and ensure that the samples are visually examined for correct labeling; and
3. Record the results of the examinations performed pursuant to subsections 1 and 2 in the applicable production or control records.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014)
Requirements for the Production of Edible Marijuana Products and Marijuana-Infused Products
NAC 453A.550 “Potentially hazardous marijuana products and ingredients” defined. (NRS 453A.370) As used in NAC 453A.550 to 453A.592, inclusive, unless the context otherwise requires:
1. “Potentially hazardous marijuana products and ingredients” means an edible item that is natural or synthetic and that requires temperature control because it is in a form capable of supporting:
(a) The rapid and progressive growth of infectious or toxigenic microorganisms;
(b) The growth and toxin production of Clostridium botulinum; or
(c) In raw shell eggs, the growth of Salmonella Enteritidis.
2. The term “potentially hazardous marijuana products and ingredients” includes, without limitation:
(a) An animal item that is raw or heat-treated;
(b) An item of plant origin that is heat-treated or consists of raw seed sprouts;
(c) Cut melons and tomatoes; and
(d) Garlic-in-oil mixtures that are not modified in a way that results in mixtures which prohibit growth.
3. The term “potentially hazardous marijuana products and ingredients” does not include:
(a) An ingredient with a value of water activity of 0.85 or less;
(b) An ingredient with a pH level of 4.6 or below when measured at 75°F (24°C); or
(c) An ingredient, in a hermetically sealed and unopened container, that is commercially processed to achieve and maintain commercial sterility under conditions of nonrefrigerated storage and distribution.
(Added to NAC by Div. of Pub. & Behavioral Health by R004-14, 3-28-2014, eff. 4-1-2014) For more information, please refer to Chapter 453A – Medical Use of Marijuana.