No products in the cart.

CALIFORNIA Marijuana Packaging and Labeling State Law
The Office of Manufactured Cannabis Safety (OMCS) has proposed the following regulations:
SUBCHAPTER 5. LABELING AND PACKAGING REQUIREMENTS Article 1. General Provisions
• 40400. Applicability.
The requirements in this section shall apply to finished cannabis products and shall not apply to cannabis or cannabis products that are transferred between licensees for purpose of further processing or packaging.
• 40401. Release to Distributor as Finished Product.
Prior to release of a product to a distributor, a licensee shall ensure that the product is in finished form and is labeled and packaged in its final form for sale at a dispensary.
Article 2. Labeling Requirements §40403. General Provisions.
(a) Any information required to be listed on a label shall be written in English.
(b) A label shall be unobstructed and conspicuous.
(c) All required label information shall be unobstructed and conspicuous.
• 40405. Primary Panel Labeling Requirements.
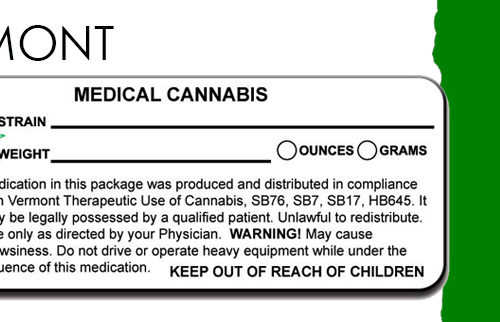
(a) The label for a cannabis product shall include a primary panel that includes the following information:
(1) The identity of the product in a text size reasonably related to the most prominent printed matter on the panel;
(2) The words “cannabis-infused” immediately above the identity of the product in bold type and a text size larger than the text size used for the identity of the product;
(3) The cannabis product symbol as prescribed in Section 40412 ;
(4) The net weight or volume of the contents of the package;
(5) The THC content and content for the package in its entirety, expressed in milligrams per package;
(6) The THC content and content per serving, expressed in milligrams per serving; and
(7) The content of other cannabinoids or terpenes per serving if such information is verified by the certificate of analysis issued by a licensed testing laboratory pursuant to Business and Professions Code section 19344.
(b) The primary panel text must be in type size no less than 6 point font and be in relation to the size of the primary panel and container.
• 40408. Informational Panel Labeling Requirements.
(a) The label for a medical cannabis product shall include an informational panel that includes the following:
(1) The licensed manufacturer and its contact number or website address;
(2) The date of manufacture;
(3) Each of the following statements:
(A) “SCHEDULE I CONTROLLED SUBSTANCE.”
(B) “KEEP OUT OF REACH OF CHILDREN AND ANIMALS” in bold print.
(C) “FOR MEDICAL USE ONLY.”
(D) “IF PREGNANT OR BREASTFEEDING, CONSULT A PHYSICIAN PRIOR TO USE.”
(E) “THE INTOXICATING EFFECTS OF THIS PRODUCT MAY BE DELAYED BY UP TO TWO HOURS.”
(F) “THIS PRODUCT MAY IMPAIR THE ABILITY TO DRIVE OR OPERATE MACHINERY, PLEASE USE EXTREME CAUTION.”
(4) A list of all product ingredients in descending order of predominance by weight or volume;
(5) If an edible product that contains an ingredient, flavoring, coloring, or an incidental additive that bears or contains a major food allergen, the word “contains,” followed by a list of the applicable major food allergens;
(6) If an edible product, the names of any artificial food colorings contained in the product;
(7) If an edible product, the amount, in grams, of sodium, sugar, carbohydrates, and total fat per serving;
(8) The lot number;
(9) Instructions for use, such as the method of consumption or application, and any preparation necessary prior to use;
(10) The product expiration date, “use by” date, or “best by” date; and
(11) The unique identifier.
(b) The informational panel text shall be in a type size of no less than 6 point font and in relation to the size of the primary panel and container, unless there is insufficient area on the container available to print all the required information in a type size of no less than 6 point font. In such a case, the label shall include the warning statements required by paragraph (3) in a type size of no less than 6 point font, and the product shall be accompanied by a supplemental labeling that includes all of the information required by this section. The text of the supplemental labeling shall be no less than 8 point font.
• 40410. Labeling Restrictions.
The label shall not contain any of the following:
(a) Claims that the manufactured cannabis or cannabis product was grown in a California county when the cannabis was not grown there.
(b) The name of a California county unless the cannabis was grown there.
(c) Content that is or designed to be attractive to individuals under the age of 21, including but not limited to:
(1) Cartoons;
(2) Any likeness to images, characters, or phrases that are popularly used to advertise to children; or
(3) Any imitation of candy packaging or labeling.
(d) False labeling information. Labeling is false if it is false or misleading in any particular.
(e) Claims of health benefits or other physical benefits.
• 40412. Cannabis Product Symbol.
The primary panel of a medical cannabis product shall be marked, stamped, or otherwise imprinted with the cannabis product symbol directly on the package.
(a) The symbol shall replicate the following in form and color:
(b) The symbol shall be no smaller in size than half (.5) inch by half (.5) inch and shall be printed legibly and conspicuously.
Article 3. Packaging §40415. Packaging.
A package used to contain a cannabis product shall adhere to the following requirements:
(a) The package shall protect the product from contamination and shall not expose the product to any toxic or harmful substance.
(b) The package shall be tamper-evident, which means that the product shall be packaged in a container within which a product is sealed so that the contents cannot be opened without obvious destruction of the seal.
(c) The package shall be child-resistant, which means the package shall be designed or constructed to be significantly difficult for children under five years of age to open or otherwise obtain access to the product contained therein within a reasonable time, and shall not be difficult for normal adults to open or obtain access to the product contained therein. A package shall be deemed child-resistant if it satisfies the standard for “special packaging” as set forth in the Poison Prevention Packaging Act of 1970 Regulations (16 C.F.R. §1700.1(b)(4)).
(d) The package shall not imitate any package used for products typically marketed to children.
(e)If the product is an edible product, the package shall be opaque.
(f) If the package contains more than one serving of cannabis product, the package shall be re-sealable so that child-resistance is maintained throughout the life of the package.
For more information please see the California Office of Manufactured Cannabis Safety’s proposed regulations.