No products in the cart.
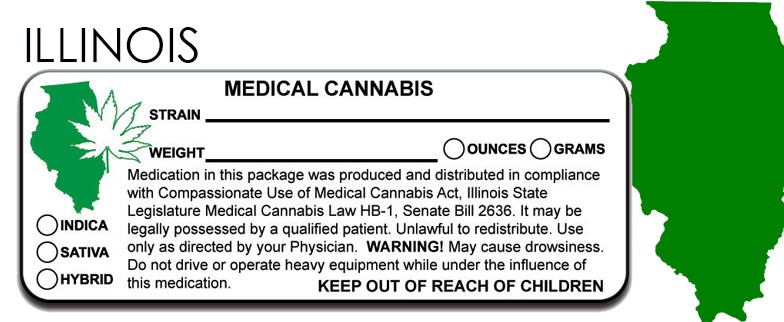
ILLINOIS Marijuana Packaging and Labeling State Law
Illinois’ cannabis labeling and packaging guidelines include the following:
• Each cannabis product produced for sale shall be registered with the Department on forms provided by the Department.
a) Each product registration shall include a label and the required registration fee (Section 1000.140). The registration fee is for the name of the product offered for sale, and one fee shall be sufficient for all package sizes.
b) All harvested cannabis intended for distribution to a dispensing organization must be packaged in a sealed and labeled medical cannabis container.
c) Packaging of any product containing cannabis shall be child-resistant and light-resistant consistent with current standards, including the Consumer Product Safety Commission standards referenced by the Poison Prevention Act.
d) Each cannabis product shall be labeled by the cultivation center prior to sale to a dispensary, and each label shall be securely affixed to the package and shall state in legible English:
• 1) The name and P.O. Box of the registered cultivation center where the item was manufactured;
• 2) The common or usual name of the item and the registered name of the cannabis product that was registered with the Department pursuant to subsection (a);
• 3) A unique serial number that will match the product with a producer batch and lot number to facilitate any warnings or recalls the Department or producer deems appropriate;
• 4) The date of final testing and packaging, if sampled, and the identification of the independent testing laboratory;
• 5) The date of manufacture and “use by” date;
• 6) The quantity (in ounces or grams) of cannabis contained in the product;
• 7) A pass/fail rating based on the laboratory’s microbiological, mycotoxins, and pesticide and solvent residue analyses, if sampled;
• 8) Content List
A) A list of the following, including the minimum and maximum percentage content by weight for subsections (d)(8)(A)(i) through (iv):
i) delta-9-tetrahydrocannabinol (THC);
ii) tetrahydrocannabinolic acid (THCA);
iii) cannabidiol ();
iv) cannabidiolic acid (A); and
v) any other ingredients besides cannabis.
B) The acceptable tolerances for the minimum percentage printed on the label for any of subsections (d)(8)(A)(i) through (iv) shall not be below 85% or above 115% of the labeled amount;
• 9) A statement that the product is for medical use and not for resale or transfer to another person.
For more information, please refer to the General Provisions for the Compassionate Use of Medical Cannabis Pilot Act.